Biography
Interests
Estael Luzia Coelho da Cruz-Cazarim1, Pâmela Santos Azevedo2, Adrielle Pereira Cordeiro3, Maiara Silva Araújo3, Damaris Salgueiro da Silva3, Matheus José Novais Landim3, Bárbara Caneschi da Costa3, Isadora Alhadas Oliveira Gomes3, Ana Karolina Toledo Fagundes3, Altacílio Aparecido Nunes4, Marina Morgado Garcia2, Marcelo da Silva Silvério5, Alessandra Ésther de Mendonça5 & Maurilio de Souza Cazarim5*
1School of Pharmaceutical Sciences of Ribeirao Preto, University of Sao Paulo, Brazil
2School of Pharmacy, Federal University of Minas Gerais, Brazil
3School of Pharmacy, Federal University of Juiz de Fora, Brazil
4Department of Social Medicine of Ribeirão Preto Medical School, University of Sao Paulo, Brazil
5Department of Pharmaceutical Sciences, School of Pharmacy, Federal University of Juiz de Fora, Brazil
*Correspondence to: Dr. Maurilio de Souza Cazarim, Department of Pharmaceutical Sciences, School of Pharmacy, Federal University of Juiz de Fora, Brazil.
Copyright © 2021 Dr. Maurilio de Souza Cazarim, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The epidemiological reach of COVID-19, emerging pandemic disease, has aroused concern among stakeholders worldwide about patients’ management. Understanding the factors that influence the management of the disease, which have not yet been fully elucidated, and understanding their associations in the planning actions to cope with disease. The aim was developed a systematic review protocol for answer the question: What clinical variables are correlated to the clinical management of COVID-19 in primary, secondary and tertiary cares? This is a systematic review protocol which has followed the Cochrane guidelines for systematic review. This protocol showed a robust method to perform a systematic review about the identification of clinical variables that are associated to progression of COVID-19. The systematic review results can aid to improve the management of COVID-19 patients at the comprehensive care in Brazil.
Systematic Review Justification
The epidemiological reach of COVID-19, emerging pandemic disease, has aroused concern among
stakeholders worldwide for two main reasons: clinical and epidemiological information on the disease is
still poor in evidence; and the influence of the health sector in the world economy, which has had a strong
impact to the point of causing one of the biggest economic recessions in history [1,2]. It is estimated that
more than 170 countries will experience a negative growth in per capita income in the year of 2020, with a
5,3% decline of the Gross Domestic Product in Brazil, which may reach 9,0% [1].
Clinical variables have been doubting object as its relevance and targeting for clinical management of COVID19. Factors related to treatment, co-morbidities, alternative therapies, can build important indicators for the fittest COVID19 care. Additionally, it is important to measure for comprehensive care in the health system and, thus to have the conception for different levels of care. In Brazil, these levels can be resumed in primary (preventive and basic care), secondary (specialized care), and tertiary care (hospital and clinicians for complex procedures) in the Public Health System [2].
In this sense, understanding the factors that influence the management of the disease, which have not yet been fully elucidated, and understanding their associations in the planning actions to cope with disease, can represent a reduction in the health systems overload and a more assertive path for the recovery of the population’s health. Fast actions and a proper management of patients infected with COVID-19 tend to soften economic impact, and this foster healthy feedback between the two sectors: health and economy [3].
Method
This protocol was registered at the base of PROSPERO - International Prospective Register of Systematic
Reviews - for systematic review, number ID 256450 (https://www.crd.york.ac.uk/prospero/). Search
strategy was performed to enhance methodological transparency and improve the reproducibility of the
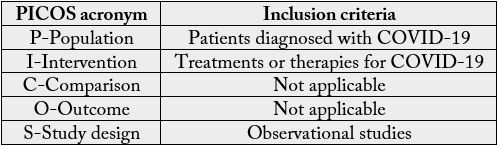
findings, following the PRISMA checklist [4]. Additionally, using the PICOS (Population/Intervention/
Comparison/Outcomes/Study Design) acronym we elaborated questions of systematic reviews, to ensure
the systematic search of scientific literature.
As COVID-19 is an emerging disease, works/articles began to be published in January 2020, so the comparator and outcomes will not be considered in the search strategy, as it is a little-known topic, described in the literature, which does not influence the specificity of systematic review, but rather improve the sensitivity [5]. Studies was retrieved from four electronic bibliographic databases: MEDLINE via PubMed, Cochrane Library by Cochrane Central Register of Controlled Trials (CENTRAL), Excerpta Medica database (EMBASE) via Elsevier and Latin American and Caribbean Health Sciences Literature (LILACS). In addition, secondary searches in other sources, such as Google Scholar will be also carried out. The reference section of the included studies will be hand-searched for additional relevant studies. The search strategy will comprise only key terms according to PICOS acronym. The studies will be selected through the following order: reading the title, abstract and full text. The data will be tabulated to list the main variables of the patients affected by COVID-19 and, thus, extract the characteristics of the study, sociodemographic characteristics and description of the intervention and control groups, if any, as well as the analyzed indicators. To access the quality of the studies, the validated Downs and Black (1998) instrument will be used, which comprises a checklist of 28 items [6]. It allows to verify the general quality of the study, the internal and external validity, bias, confusion, and the power of the analyzes. This measurement is performed by a generated score and can be applied to observational studies [6], which is interesting due to the need addressed in this systematic review and the openness given by the journals to this pandemic for publication on this topic. The selection will be carried out with the aid of the Rayyan platform for systematic reviews [7].
The structuring of this systematic review, based on the Cochrane guide for systematic review and metaanalysis, progressing in the stages of search, selection, data extraction, compilation and analysis and results [5].
What clinical variables are correlated to the clinical management of COVID-19 in primary care, secondary
care and tertiary care?
A literature search will be carried out with the need to establish a pattern of the main influential variables for
the epidemiological scenario of COVID-19, also meet a demand from the Brazilian Public Health System
and generate synthesis of evidence [2].
The objective is to understand and relate qualitatively the different variables that have been associated with
the clinical management of the COVID-19 for creating of a patient management algorithm in the public
health system in Brazil.
The condition or the study domain will be defined for the management of COVID-19 developed in primary,
secondary and tertiary health care. In addition, the treatments proposed, and respective clinical variables
analyzed will be relevant to the domain of this themes [3].
The definition of research categories is based on two main categories for clinical trials (PICO) according to Higgins and Green (HIGGINS, GREEN, 2011) [7]. A summary of the Population (P), Interventions (I), Comparators (C) and Outcomes (O) considered, following the PICO acronym, is shown in table 1.
The Boolean operators to be used are: intra-category “OR” for word combination and, inter-category “AND” for word combination, population and intervention. No search filters will be used. It is emphasized that the search groups should be divided according to PIC, and when combining the “OR” the terms/words should always be in parentheses because unlinking the “OR” in the search may find off-target results and provide a broad, inaccurate search. Descriptors and keywords were extracted from controlled vocabularies, Emtree thesaurus for the strategies assembled in EMBASE, Health Science Descriptors (DeCS) in Portuguese/ Spanish/English for LILACS; MeSH (Medical Subject Headings) for PubMed, Cochrane e Scopus. The search was run on February 25th, 2021, in English, Portuguese and Spanish. The search strategies were adapted to each search base according to its peculiarities (Table 2).
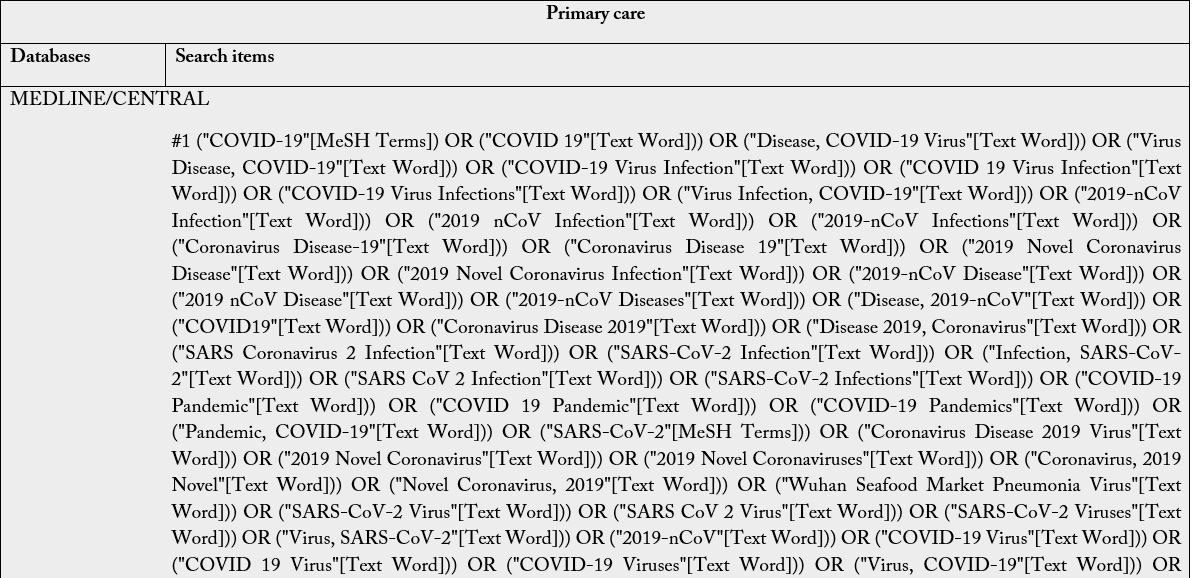

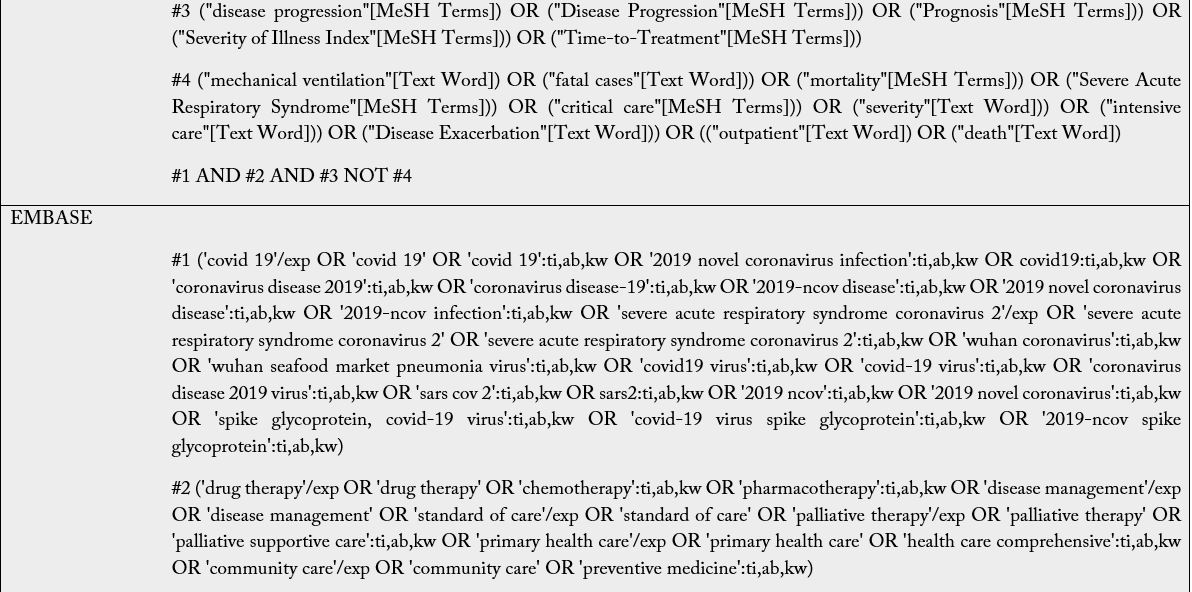
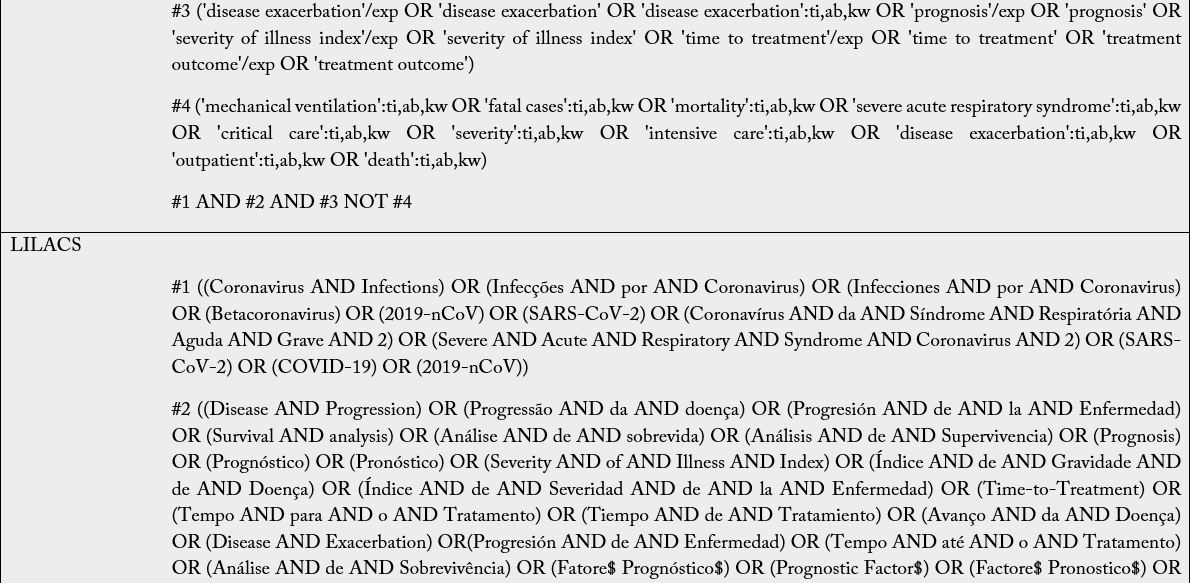

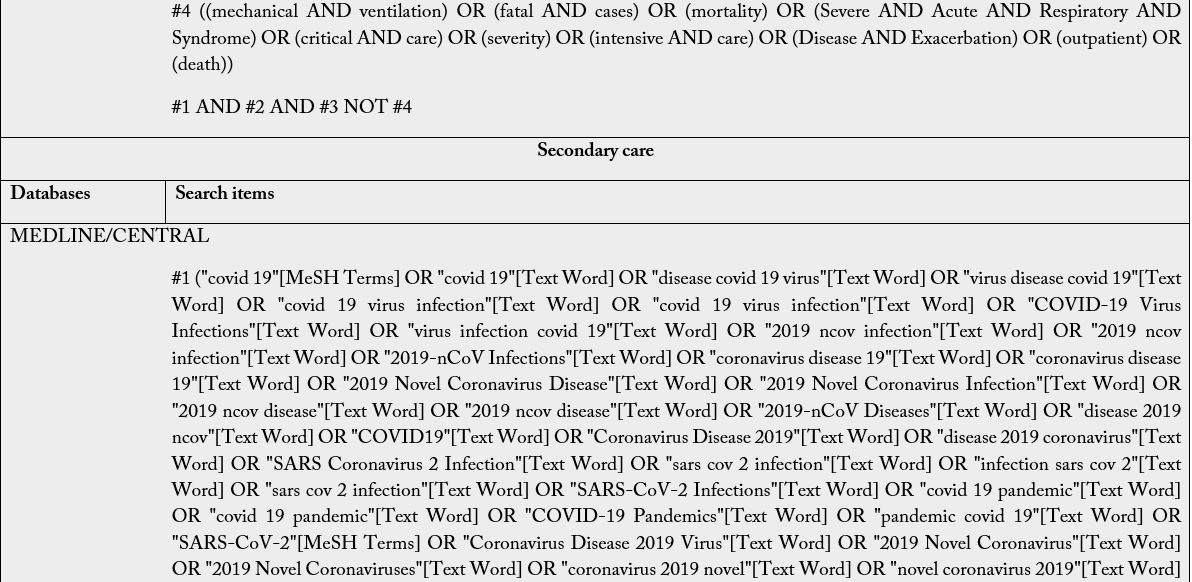

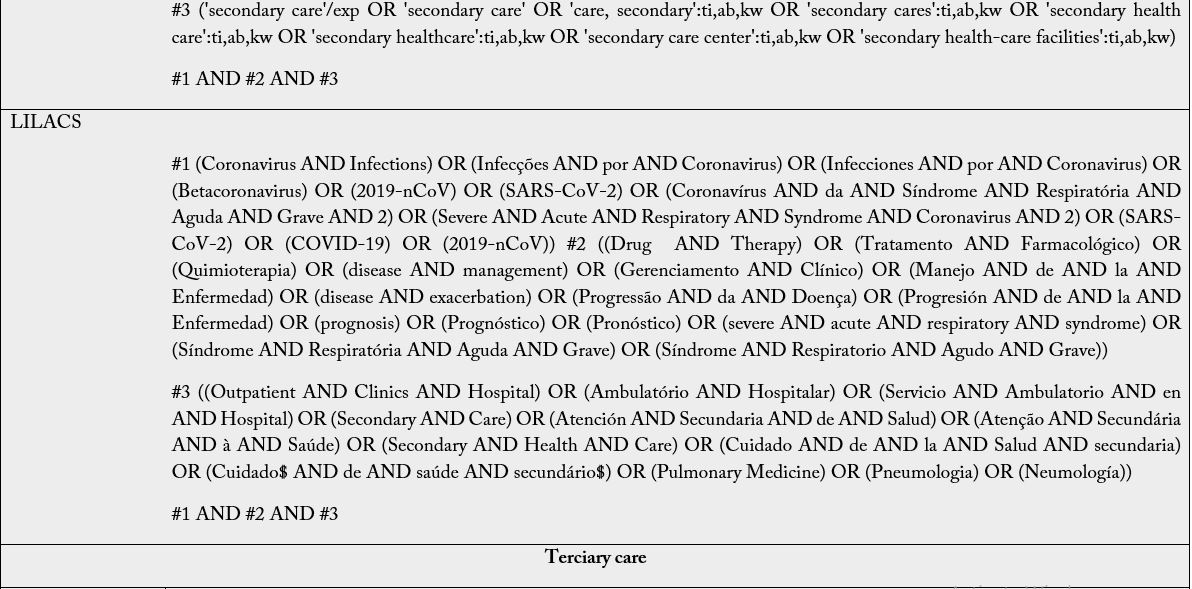

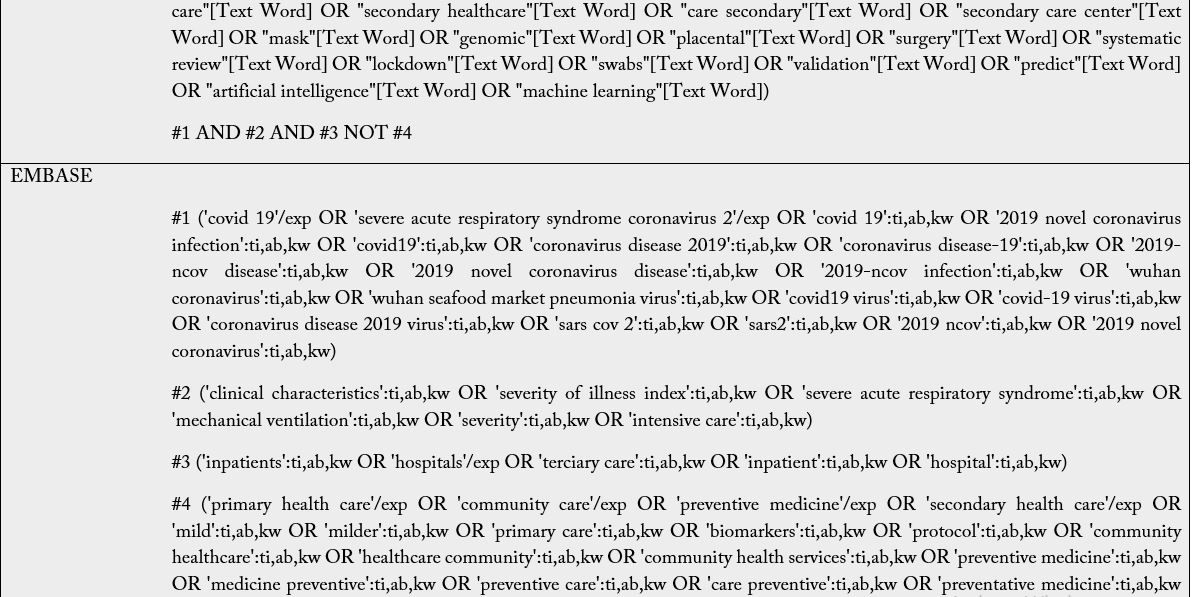
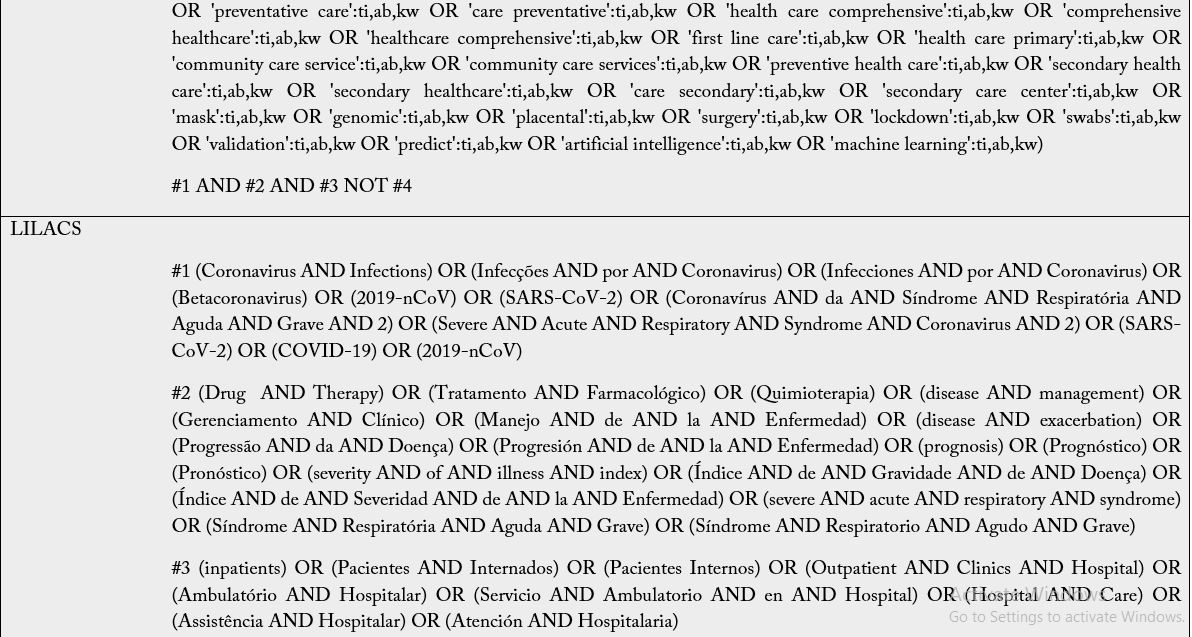

Subtitle: MEDLINE, Medical Literature Analysis and Retrieval System Online; CENTRAL, Cochrane Central Register of Controlled Trials; EMBASE, Excerpta Medica Database; LILACS, Latin American and Caribbean Health Sciences Literature. For The LILACS, it must be used the DeCS and, of these descriptions/ keywords, the translation into the Portuguese and Spanish from the dictionary should be used when searching for words in the various fields.
The selected articles after reading the title and abstract will be fully analyzed to be included those studies that answer the question of the systematic review and that meet with the inclusion, exclusion, and eligibility criteria. Conflicts of article selection will be mutually resolved, when it is not agreed by the authors, a third researcher will have the decision-making power. In addition, original articles will be considered and limited to the languages english, spanish and portuguese. It should be noted that clinical trials will be analyzed in a separate group from other studies, and divided between randomized and non-randomized. Additionally, meta-analyses and systematic reviews will not be included in the results of the review, since this would require another course for the systematic review. The following inclusion and exclusion criteria are listed.
Studies with patients diagnosed with COVID-19 on pharmacological or non-pharmacological treatment
for COVID-19 with the clinical management of COVID-19 at primary, secondary and tertiary levels of
health care.
Studies that did not consider the treatment of patients diagnosed with COVID-19; studies that analyze
variables that are not reproducible for interventions regarding the clinical management of COVID-19;
studies that do not point out variables correlated to the care of the patient with COVID-19; the variables
described in the clinical management do not have clarity regarding their correlation with the disease; studies
of narrative or integrative reviews, dissertations or theses, editorials, news, comments, letters to the editor,
abstracts published in proceedings of scientific journals or congresses, and guidelines; studies that do not
contemplate the PICO strategy.
It’s important to know that this review was divided into three systematic reviews. It is important to know
about clinical variables that have influences on patients’ final outcomes. They must be summarized differently
at primary, secondary and terciary cares. In this sense, the studies must bring results that show the association
among clinical variables and outcomes for COVID-19 patients.
The effect will be measured by Relative Risks for experimental studies and odds ratio for observational
studies reasoned in extracting those significant variables from selected studies in this review.
The eligibility of the studies found will be analyzed for the studies included in the review and will follow the criteria according the previously prepared checklist:
1. Evaluator’s name;
2. Study ID:
• Author, title and reference.
3. Eligibility requirements:
• Verification of all items described in the inclusion criterion;
• Biases that compromise the study due to potential conflicts of interest;
• Availability of data to be included in the systematic review.
4. Eligibility confirmation:
• Considering the course of the study, the intervention, the people involved, the study has potential to be
included (The study’s course is stated and consistent with the methods);
• Participants consistent with the purpose of the study; Interventions considered are consistent with the
study’s proposal;
• The evaluated results are consistent with the study’s proposal)? (Yes or No)
The data synthesis and tabulation will occur in such a way that the quantitative and qualitative data have been extracted from the studies included in the review using the data form developed by the reviewers (excel table template). After the end, the collaborators will assist in the discussion and interpretation of the analyzed data for the set of variables.
Initially, the bias assessment will be performed using the Cochrane guideline of bias risk classification [5].
The risk of bias assessment will be evaluated for clinical trials and observational studies in order to access the
quality of the study, Downs and Black instrument will be used, that makes up a 28-item checklist (allows
to check the overall quality of the study, the internal and external validity, biases, confounding and power of
the analyses).
Stage of Data Extraction
● Identification of clinical variables associated with the clinical course and management of COVID-19;
● Assemble the Excel spreadsheet with the categories of variables selected added in columns;
● Start the data extraction as:
o Fundamental variables:
1. Year
2. Reference
3. Country
4. Author
5. Planned sample number
o Details of the methods:
1. Type of study
2. Study design (how many groups; mode of data analysis)
3. Follow-up time
4. Randomization (“adequate”, “inadequate” or “not reported” by the study)
5. Confidential allocation (reported or unreported)
6. Blinding scheme:
• Investigators - “yes”, “no” or “not reported”
• Participants - “yes”, “no” or “not reported”
• Outcome evaluators - “yes”, “no” or “not reported”
o Participants:
1. Number of patients randomized by treatment mode or divided between comparison groups (exposed and
unexposed)
2. Number of follow-up losses per group
o When clinical trials: clinical protocol
● Study phase
● Type of screening (inclusion criteria and sample planning)
● Assessment of safety and/or effectiveness
● Gold standard comparator (placebo or standard treatment? Which one?)
● Others.
o Health care level:
1. Primary or preventive
2. Emergency
3. Hospital: Outpatient
4. Hospital: Hospitalization
5. Hospital: ICU admission COVID-19
o Treatment
● Self-isolation only
● Basic medication + Self-isolation
● Basic medication + exams + hospitalization
● Oxygen therapy
● Intubation + sedation
● Renal Therapy
● High technological density procedures
o Outcomes
● Definition of each outcome investigated given the clinical condition of the study population;
● Unit of measure (if applicable) used as the gold standard (yes or no)
• Define death or survival
• Define disability or sequela criteria
• Perfect health, as before the disease
o For scales:
• report the upper and lower limits;
• report how high or low the score is and whether there has been clinical improvement
● Results
o For each outcome, report categorical and/or continuous and/or discrete variables.
o Calculate the rate of remission or recovery (control of clinical condition)
1. Sociodemographic descriptive data:
• Average age
• Percentage of sex
• Average income
• Education
• Ethnicity
• Previous disease history
• Comorbidities
• History of health events associated with CNCD
2. Clinical parameters of clinical interest:
• Staging the population’s risk
• Obesity
• Smoker
• Alcoholic
• Types of pre-existing diseases
Others
Before the moment of consensus between the two researchers, the agreement between them will be analyzed
by the Kappa coefficient, with a value above 0.80 being acceptable, otherwise, there will be a need to
restructure a new search strategy. The Mann-Whitney test will be performed to test the difference between
the quality scores of the studies with favorable and unfavorable clinical results. Other statistics will be used
according to the behavior of the variables to be analyzed.
● Analysis of subgroups or subsets: The extraction of data for each level of care will be planned together, which will allow the listing of important subgroups or sets to be analyzed.
Correlation degree of the clinical variable to the progression or remission of COVID-19.
● Primary outcomes
Correlation degree of the clinical variable to the progression or remission of COVID-19.
● Secondary outcomes
Patients’ features or co-variables associated with the progression or remission of COVID-19.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!